INTRODUCTION
Stress urinary incontinence (SUI) is defined by the International Continence Society (ICS) as the involuntary loss of urine with force or physical exertion and sneezing or coughing. The etiology of SUI is multi-factorial and includes urethral weakness, with or without damage to the striated muscles, disrupted pudendal nerve innervation, loss of pelvic floor support, and urethral hypermobility. SUI is often attributed to injury during pregnancy and childbirth, but can also be associated with body mass index (BMI), chronic constipation, pelvic surgery, neurologic diseases and family predisposition [
1].
Pelvic floor muscle exercises (PFMEs) have been the first-line treatment for urinary incontinence since Arnold Kegel introduced PFMEs 50 years ago [
2,
3]. In fact, PFMEs are highly effective when performed properly and conscientiously [
2,
3]. Significant improvement of muscle function requires proper instruction, and regular and persistent exercise lasting for at least several months [
4]. Nonetheless, studies have shown that approximately 30% of patients are unable to perform isolated pelvic floor contractions by following written or verbal instructions [
5]. Rather than relying on voluntary PFMEs, it may be possible to achieve the same trophic effects on muscle fibers by activating muscles with neuromuscular electrical stimulation (NMES). Intramuscular electrodes can excite the terminal branches of motor axons causing little or no sensation other than direct stimulation of the muscles
per se [
6,
7].
The NuStim
® system is a newly developed stimulation tool (an implantable wireless driver microstimulator) designed by Chengnuo Ltd. (NuStim device; GenralStim Inc., Zhejiang, China). The microstimulator targets NMES during PFMEs. The system consists of three major subsystems (Fig.1): an implanted microstimulator for chronic intramuscular electrical stimulation, a radio-frequency (RF) external transmitter in a seat cushion; and a remote control via an Android smart-phone/tablet app. The NuStim
® system is user-friendly and mobile, allowing for flexibility during PFME training schedules [
8]. The aim of this study was to test the utility and safety, of the clinical system and its software application when used by physicians and patients in a real-world clinical trial.
MATERIALS AND METHODS
Study Design
The study was a prospective, non-blinded, self-controlled pilot study conducted from October 2017 to October 2019 at the China Rehabilitation Research Centre (CRRC) of Capital Medical University (CMU), Beijing, China. Ethical approval was obtained from the CRRC Ethics Committee (MW-QX-02-2016). Patients with SUI were recruited from urology, gynecology, and primary care clinics. The exclusion criteria included those who (1) had previously received incontinence surgery; (2) was currently on concomitant medical treatment for urinary incontinence; (3) had urinary tract infections and neurologic or psychiatric diseases. All the participants provided written informed consent. All of them underwent a standardized assessment by a 1-hour pad test (1-h pad test) as the primary outcomes measurement, the international consultation on incontinence questionnaire short form (ICI-Q-SF), and the patients’ perception of bladder condition (PPBC) to assess the effect of UI on quality of life (Qol), followed by a physiotherapist-conducted pelvic floor muscle strength assessment using the modified Oxford scale (MOS) to assess the suitability for planned treatment and collection of baseline data.
Device
The percutaneously implantable and wireless microstimulator (NuStim®) is of cylindrical shape, with 2 leads on each side (3 mm in diameter ×10 mm in length) (Fig. 2A). The NuStim® system can be implanted into the pelvic floor muscles by employing a package of insertion tool (Fig. 2) to elicit strong contractions without producing unpleasant sensations and without requiring any voluntary exertion. The stimulator receives RF electromagnetic pulses from an external source, the RF cushion (Fig. 1), and transforms the RF electromagnetic pulses into electrical pulses. The stimulation settings were as follows: 0-5 V, 2-20 Hz pulse rate and 200 ms pulse width. The electrical pulses generated by the stimulator were biphasic, asymmetric and exponential. The stimulation parameters can be adjusted during the treatment remotely via an app on an Android pad.
The stimulator can be implanted and put in place by utilizing a sterile NuStim® insertion tool, consisting of an electrode needle ensheathed in a dilator and a disposable hand-held stimulator (Fig. 2B). The NuStim® implantation was performed under local anesthesia with the patient assuming the lithotomy position. Ideally, the stimulator should be implanted close to the external urinary tract sphincter (usually 1.5 cm away from the anal orifice) so that the stimulator can induce contractions of pelvic floor muscles (Fig. 3A). A low threshold implantation site is first located using a disposable hypodermic EMG needle connected to the hand-held stimulator via a pinjack adapter (Fig. 3B). A circuit electrode is connected to the back of the hand-held stimulator and attached to the skin. A skin incision was made at a different location as an entry for the NuStim® insertion tool, which was about 1 cm away from anal orifice and approximately perpendicular to the perineum. The insertion tool, with its needle electrode attached to the hand-held stimulator, was advanced, 1-cm each time, toward the target site (i.e., terminal branches of motor axons). The threshold for inducing visible contractions decreased as the needle electrode approached the motor axons, and then increased, thereby the lowest threshold could be identified (Fig. 3C). At this point, the stimulator with needle electrode and dilator was withdrawn, with the sheath staying in place. The NuStim® was inserted into the sheath with the cathode facing the tissue (Fig. 3D). The needle and dilator were used to push the NuStim® through the sheath to the tapered end until it was snugly fit with some resistance. When the needle on the stimulator connects to the back of the NuStim implant, the stimulation pulses pass through the implant to the cathode of the electrode, which was used to confirm the aforementioned lowest threshold. The NuStim® was ultimately released to its site and the sheath is retracted over the dilator.
Treatment Protocol
Initial training started 1 week after NuStim® implantation. During the initial training under the supervision of a physiotherapist, the system was activated and the subject received PFME training in sitting position according to the standard protocol. The stimulation threshold level was based on identification of the first twitch sensation at the lowest stimulus. The target intensity of stimulation was identified when the strongest twitch occurred or the most comfortable level was reached, whichever came first. After the intensity was identified, stimulation cycle parameters were then selected to provide strong, cyclical contractions and relaxations for the desired exercise period (typically 30‒60 min/day). The stimulation parameters were individually set according to each participant’s conditions. Participants were encouraged to contract the corresponding pelvic floor muscles to the stimulation rhythm and do daily training at home (5 days per week for 13 weeks, or 25 weeks if the participant decided to continue). The 1-h pad test was conducted at week(s) 0, 1, 3, 8, and 13 by an evaluator, if the participant decided to continue training, and then every 6 weeks thereafter until the 25th week (Fig. 4). The questionnaire evaluation and pelvic floor muscle assessment were completed before training at week(s) 0, 13, and 25 by a doctor and a physiotherapist, respectively.
Outcome Measures
The primary outcomes for this study were SUI status as measured by the 1-h pad test, with secondary outcomes being pelvic floor muscle strength, ICI-Q-SF, and PPBC scores. The evaluations were conducted before and after treatment by one non-blinded physiotherapist and one non-blinded investigator. For the pelvic floor muscle assessment, our physiotherapist used the MOS to measure muscle strength on a 6-point Likert-type scale (0 = no contraction; 1= flicker; 2 = weak; 3= moderate [with lift]; 4 = good [with lift]; 5 = strong [with lift]).
The participants were asked to perform three maximum voluntary contractions and the best voluntary contraction was taken and was scored. Both groups were encouraged to perform daily PFMEs and record exercise frequency. At the end of the treatment, the participants were asked about their perceived improvement in urinary leakage. The participants were asked to give feedback about the treatment results (unchanged or improved), and if improvement was reported, the participants were asked if they considered themselves continent. The participants with no improvement were followed up in a urology clinic and offered different treatment options.
Statistical Analysis
All of the statistical analyses were performed using a commercially available statistical software package (Graph Pad Prism version 5.0.3; Graph Pad Software, San Diego, CA). The 1-h pad test data was normalized to the baseline control data in each experiment. The normalized data from different patients were presented as mean ± SD. ANOVA followed by Dunnett’s multiple comparison test and Student’s t-test were used to detect statistical significance (P < 0.05).
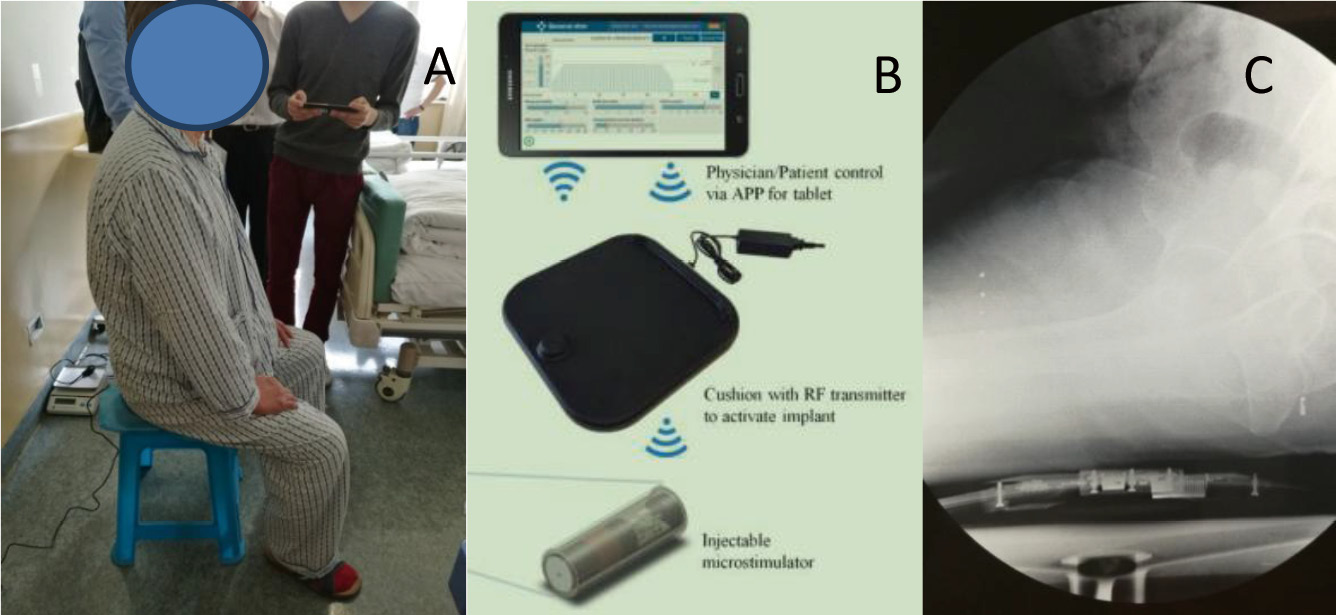
Figure 1. Practice of NuStim operation. A. The patient receives passive exercise while sitting on the RF-Cushion and performing daily activities; B. Exercise is adjusted on a tablet and transmitted wirelessly to the RF-Cushion which wirelessly motivates and controls the implanted microstimulator; C. X-ray showed the position relative (sagittal) of the microstimulator (in vivo) and the RF-Cushion.

Figure 2. Assembled NuStim insertion tool. A. The injectable microstimulatior; B. The dilator (3.26 mm o.d. x 117.6 mm length) passes through the sheath (4.27 mm o.d.).
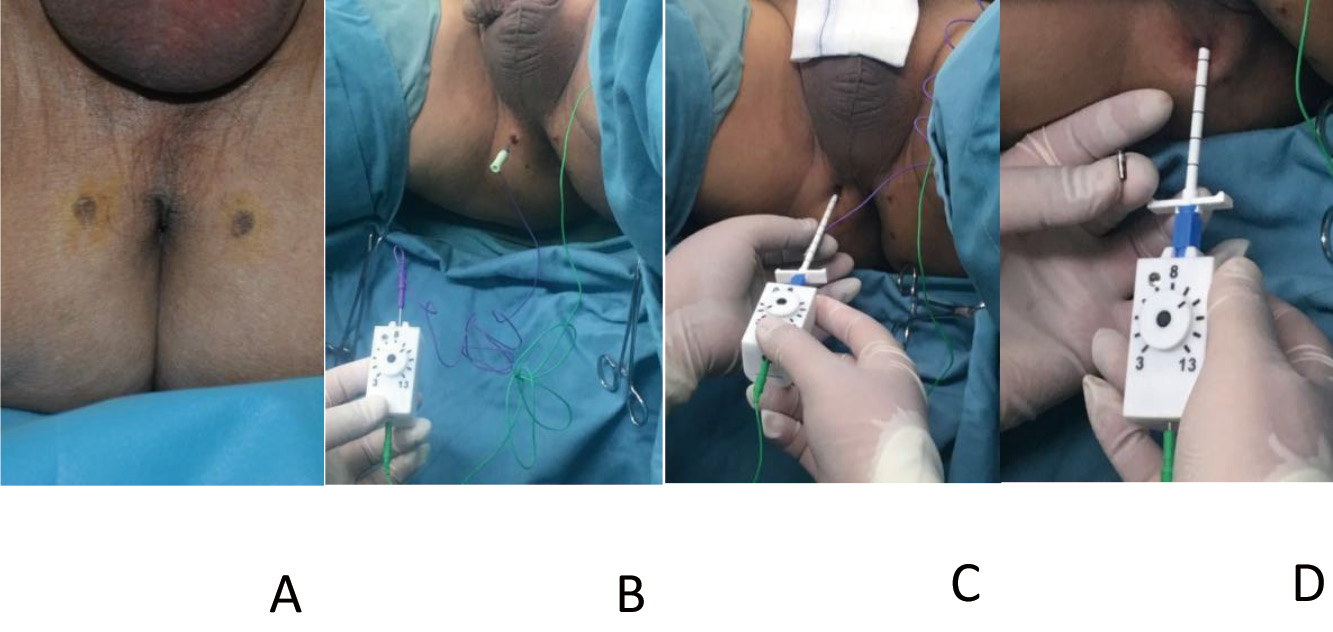
Figure 3. NuStim injectable microstimulatior implantation and deployment. A. The location for the stimulator implantation; B. A low threshold implantation site was obtained; C. The insertion tool with its needle electrode and handheld stimulator; D. The NuStim is placed into the sheath with cathode facing the tissue. A. The ideal location for the stimulator implantation is close to the external urinary tract sphincter so that it can generate pelvic floor muscle contraction determined above (usually 1.5 cm to the parallel outside of anal orifice); B. A low threshold implantation site is first located using a disposable hypodermic EMG needle connected to the handheld stimulator via a pinjack adapter; C. The insertion tool with its needle electrode attached to the handheld stimulator is advanced in 1 cm steps toward the target site. The threshold for inducing visible contractions could be identified; D. The NuStim is placed into the sheath with cathode facing the tissue.
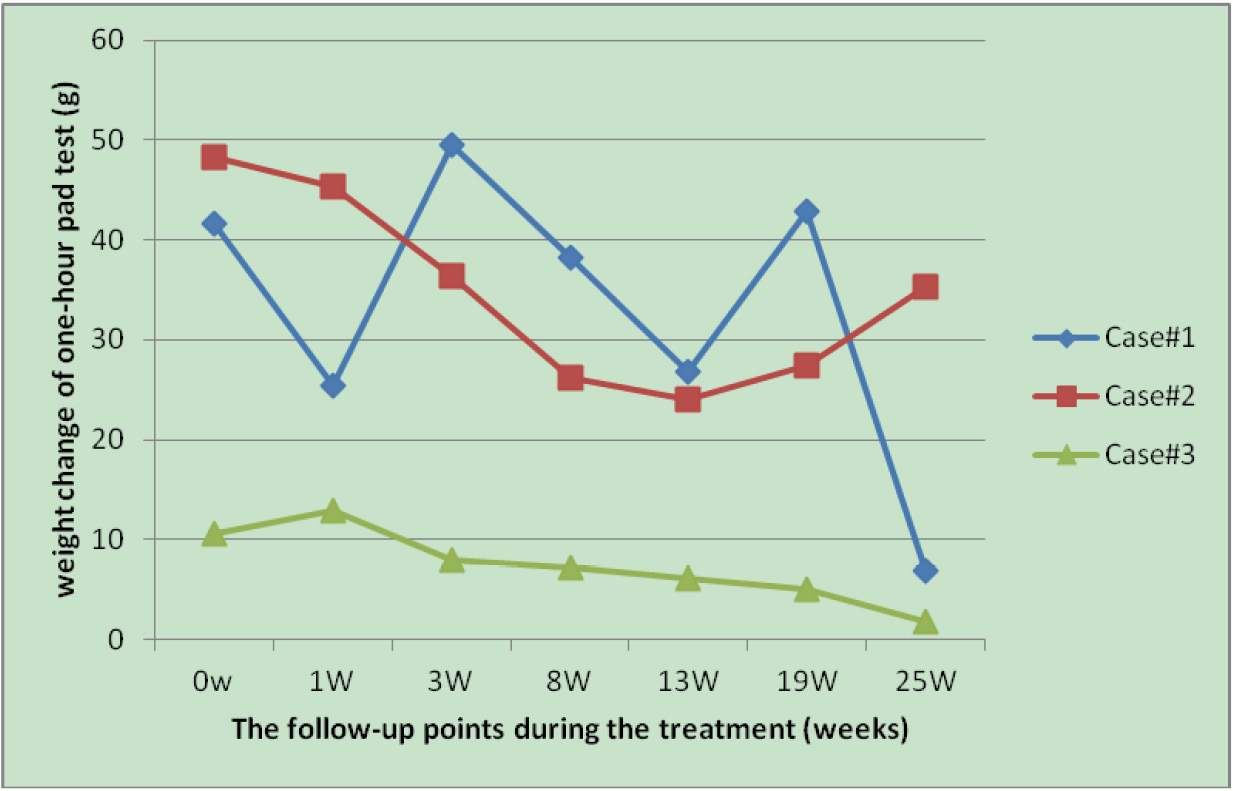
Figure 4. The trend of the weight change of one-hour pad test at baseline and during the treatment, the demographic characteristics and etiological classification of patients with stress urinary incontinence at time of first referral. Case 1: Male, 75 yrs., 2 yrs. after RP, severe SUI as assessed by 1H-pad test weight gained over 40 g at baseline. Stimulation parameters: 20 Hz, Level 10, 4 S on, 5 S off; Case 2: Male, 57 yrs., 1 yr. after TURP, severe SUI as assessed by 1H-pad test weight gained over 40 g at baseline. Stimulation parameters: 4 Hz, Level 7, 4 S on, 5 S off;Case 3: Female, 47 yrs., 5 yrs. of moderate-severe SUI as assessed by 1H-pad test weight gained around 10 g at baseline. Stimulation parameters: 15Hz, level 20, 4 S on, 5 S off.
RESULTS
Three participants were recruited with no dropouts, including 2 post-prostatectomy incontinence (PPI) subjects and 1 female SUI patient. All of the participants received regular pelvic floor muscle training during the 25-week treatment period, and there was no significant difference in the training frequency among the participants. All of the three participants had finished the 25 weeks PFMEs treatment protocol, and there was no side effect reported during the training period.
The NuStim® treatment was shown to be capable of alleviating SUI, as assessed by normalized weight of the 1-h pad test, which presented a significant linear trend (P = 0.0021). Intragroup analysis showed that all participants achieved statistically significant improvement in terms of the 1-h pad test score at 25 weeks compared with pre-training status as revealed by Dunnett's multiple comparison test (Fig. 4). However, there was no significant difference between other time points during the follow-up compared to the pre-treatment baseline (P = 0.058). Comparison of the secondary outcomes, such as ICI-Q-SF, PPBC, and MOS scores, for each participant demonstrated no significant differences at the conclusion of the study (Table 1).
Table 1 Comparison of the secondary outcome variables ICI-Q-SF(a), PPBC(b) and MOS(c) score before and after treatment.
|
|
|
|
|
|
|
|
|
|
| |
a |
b |
c |
a |
b |
c |
a |
b |
c |
| Case1 |
17 |
4 |
3 |
15 |
4 |
3 |
13 |
1 |
3 |
| Case2 |
13 |
4 |
3 |
13 |
4 |
3 |
13 |
4 |
3 |
| Case3 |
10 |
4 |
4 |
9 |
3 |
4 |
9 |
3 |
4 |
There was no significant difference in the secondary outcome variables, such as ICI-Q-SF, PPBC and MOS scores in each participant at the end of study. ICI-Q-SF: international consultation on incontinence questionnaire short form; PPBC: patient perception of bladder condition; MOS: modified Oxford scale.
DISCUSSION
After 25 weeks of treatment, participants attained significant alleviation in SUI symptoms, as shown by improved weight of 1-h pad test. This result was in agreement with the Cochrane meta-analysis comparing the effects of pelvic floor muscle training with that of no treatment [
4]. Secondary outcome variables (self-reported score) were not significantly different after 25 weeks of training, although the participants still had better PPBC scores by the end of the study. It is noteworthy that the Nustim® provided patients with an easy and accurate means to know which pelvic floor muscles needed to be contracted, thus enhancing the effectiveness of pelvic floor training. This treatment may help to improve the pelvic muscle tone and neuromuscular function, thus enhancing urethral closure mechanism when intra-abdominal pressure is increased.
So far, there is no standardized outcome measure for urinary incontinence. The ICS recommends that urinary leakage be used to evaluate treatment effects. We used the 1-h pad test as a primary outcome measure in this study instead of the 24-h pad test or leakage episodes because the short pad test (1 h) is easy to perform for daily follow-up. A Cochrane meta-analysis reported that the pad test outcomes did not coincide with the actual data on leakage episodes, and variability in the findings restricted the use of pad test results in the comparison of data among different studies [
4,
9]. Thus, more accurate evaluation of SUI should be performed in future studies regarding this biofeedback device.
It is noteworthy our study enrolled three participants (two PPI participants and one female SUI participant). The PPI participants had severe SUI, while the female participant had mild-to-moderate SUI. The female participant accomplished a better treatment effect compared to her male counterparts. This finding may be attributed to the etiology of SUI and the anatomic difference between genders. The value of the various approaches for conservative management of PPI after radical prostatectomy (RP) remains uncertain. It seems unlikely that men benefit from one-on-one pelvic floor muscle training after transurethral resection of the prostate (TURP) [
9]. In our opinion, the PPI participants with moderate-to-severe symptoms may suffer from SUI due to intrinsic sphincter deficiency (ISD) that requires urologic surgery, such as artificial urinary sphincter or male sling implantation [
10].
Subjective measures of the severity of incontinence and the impact of SUI on the quality of life (QoL) are important in the evaluation of urinary incontinence. In our study, ICI-Q-SF and PPBC scores were used as second outcome measures because the scoring systems consider urge incontinence or overactive bladder symptoms, whereas our study focused on SUI symptoms. The self-reported measures, such as ICI-Q-SF and PPBC scores, were better at the end of follow-up, although the ICI-Q-SF and PPBC scores were not significantly improved or changed. This finding could be a consequence of a small sample size. It is important to note that the QoL improvement recorded in SUI participants with PFMEs was mainly due to the ease of using the device, which led to a better compliance rate and results. Compliance with treatment plan is important in muscle strength training because the results depend heavily on regular training. All of our participants complied with the Nustim® training schedule, since the participants found the portable device convenient to use in daily life.
A potential weakness of this study was a relatively small sample size, leading to a lack of power to detect subtle associations. Long-term PFMEs are necessary for maintaining the treatment effect, and other treatment methods may be needed in more severe SUI patients and/or special populations.
CONCLUSIONS
This study showed that the NuStim® is a good adjunct to PFMEs, as the NuStim® helps patients benefit more from pelvic floor muscle training. NuStim® is a small, portable device that can be conveniently used on daily basis to perform effective PFMEs. However, further studies are warranted to reach a definite conclusion about its efficacy.