Sex differences in the MB49 syngeneic, murine model of bladder cancer

Objective: The MB49 syngeneic, murine model of bladder cancer has been widely used for more than 35 years. In humans, bladder cancer is one third as prevalent in women as in men, with a trend toward lower prevalence in parous compared to nulliparous women. Our objective was to determine if the MB49 bladder cancer model reproduces the sex differences observed in humans, and to determine its sensitivity to testosterone and the pregnancy hormone, human chorionic gonadotropin (hCG).
Methods: Male and female C57BL/6 mice were implanted with MB49 murine bladder cancer cells, and observed for tumor growth. MB49 dose responses to hCG and dihydrotestosterone were determined in vitro.
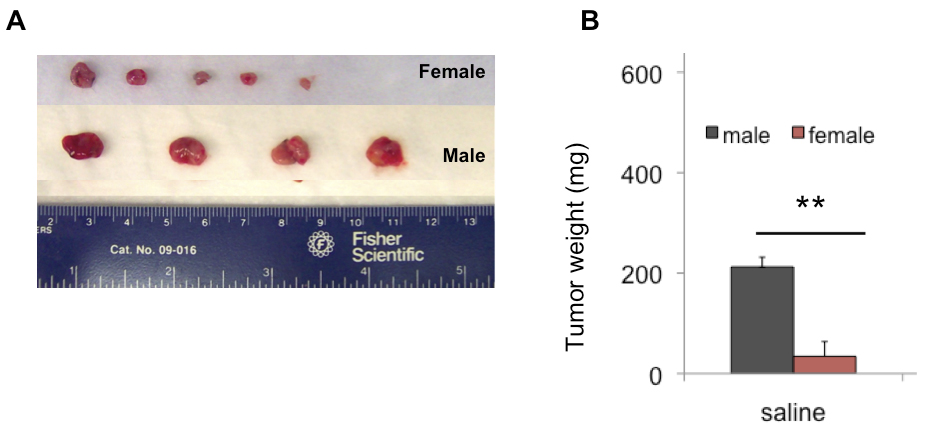
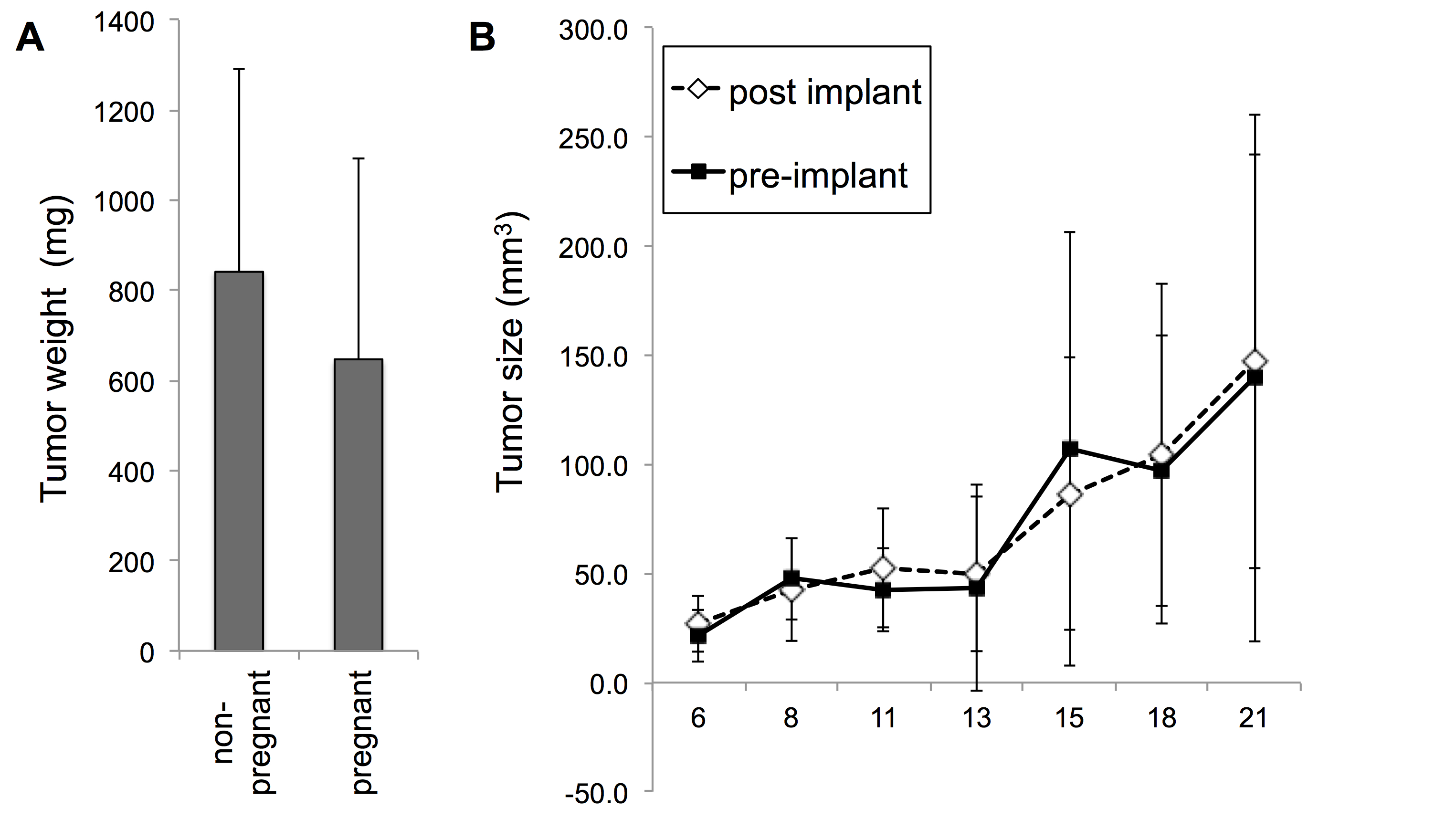
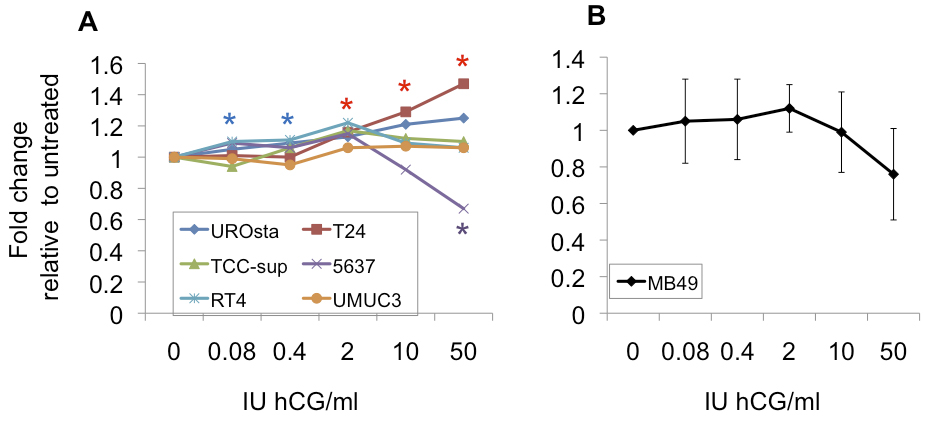
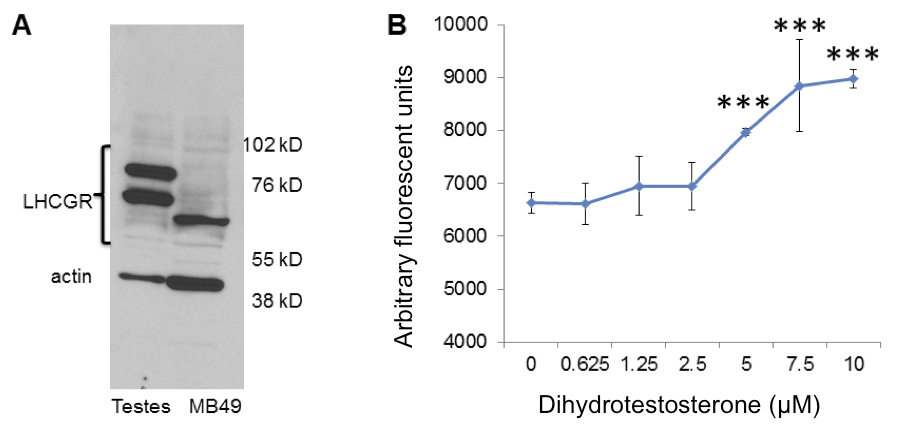
Results: MB49 tumor growth was significantly greater in male mice than female mice. Pregnancy did not affect MB49 tumor growth in female mice. MB49 cells did not proliferate in response to hCG in vitro and the functional receptor for gonadotropins was absent. Dihydrotestosterone strongly stimulated growth of MB49 cells in vitro.
Conclusions: The MB49 murine model of bladder cancer reproduced some aspects of the sex differences observed in humans. Our results suggest that testosterone may stimulate MB49 cell proliferation, which may explain the more rapid MB49 tumor growth observed in male mice.
Introduction
Materials and Methods
Mice
Cells and reagents
Tumor implantation and hCG treatment
MTS assay
Western blot detection of hCG receptor with LHCGR antibody
Dihydrotestosterone dose curve growth assay
Statistical analysis
Results
MB49 tumor growth is greater in male than female mice
Concurrent pregnancy did not inhibit MB49 tumor formation
hCG treatment does not stimulate MB49 bladder cancer cell proliferation in vitro
MB49 cells grow in response to dihydrotestosterone in vitro
Discussion
Conclusions
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973-2012), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2015, based on the November 2014 submission.
- Hartge P, Harvey EB, Linehan WM, Silverman DT, Sullivan JW, et al. (1990) Unexplained excess risk of bladder cancer in men. J Natl Cancer Inst 82: 1636-1640. [PubMed] [Google Scholar]
- Castelao JE, Yuan JM, Skipper PL, Tannenbaum SR, Gago-Dominguez M, et al. (2001) Gender- and smoking-related bladder cancer risk. J Natl Cancer Inst 93: 538-545. [PubMed] [Google Scholar]
- McGrath M, Michaud DS, De Vivo I (2005) Hormonal and reproductive factors and the risk of bladder cancer in women. Am J Epidemiol 163: 236-244. doi: 10.1093/aje/kwj028. [View Article] [PubMed] [Google Scholar]
- Daugherty SE, Lacey Jr JV, Pfeiffer RM, Park Y, Hoover RN, et al. (2013) Reproductive factors and menopausal hormone therapy and bladder cancer risk in the NIH-AARP Diet and Health Study. Int J Cancer 133: 462-472. doi: 10.1002/ijc.28022. [View Article] [PubMed] [Google Scholar]
- Summerhayes IC, Franks LM (1979) Effects of donor age on neoplastic transformation of adult mouse bladder epithelium in vitro. J Natl Cancer Inst 62: 1017-1023. [PubMed] [Google Scholar]
- Fabris VT, Lodillinsky C, Pampena MB, Belgorosky D, Lanari C, et al. (2012) Cytogenetic characterization of the murine bladder cancer model MB49 and the derived invasive line MB49-I. Cancer Genet 205: 168-176. doi: 10.1016/j.cancergen.2012.02.002. [View Article] [PubMed] [Google Scholar]
- Böhle A, Jurczok A, Ardelt P, Wulf T, Ulmer AJ, et al. (2002) Inhibition of bladder carcinoma cell adhesion by oligopeptide combinations in vitro and in vivo. J Urol 167: 357-363. [PubMed] [Google Scholar]
- Rossi MR, Masters JR, Park S, Todd JH, Garrett SH, et al. (2001) The immortalized UROtsa cell line as a potential cell culture model of human urothelium. Environ Health Perspect 109: 801-808. [PubMed] [Google Scholar]
- Tomayko MM, Reynolds CP (1989) Determination of subcutaneous tumor size in athymic (nude) mice," Cancer chemotherapy and pharmacology. Cancer Chemother Pharmacol 24: 148-154. [PubMed] [Google Scholar]
- Maston GA, Ruvolo M (2002) Chorionic gonadotropin has a recent origin within primates and an evolutionary history of selection. Mol Biol Evol 19: 320-335. [PubMed] [Google Scholar]
- Zaravinos A, Lambrou GI, Boulalas I, Delakas D, Spandidos DA (2011) Identification of common differentially expressed genes in urinary bladder cancer. PLoS One 6: doi: 10.1371/journal.pone.0018135. [View Article] [PubMed] [Google Scholar]
- Vergara GJ, Irwin MH, Moffatt RJ, Pinkert CA (1997) In vitro fertilization in mice: Strain differences in response to superovulation protocols and effect of cumulus cell removal. Theriogenology 47: 1245-1252. [PubMed] [Google Scholar]
- Wang XN, Greenwald GS (1993) Human chorionic gonadotropin or human recombinant follicle-stimulating hormone (fsh)-induced ovulation and subsequent fertilization and early embryo development in hypophysectomized fsh-primed mice. Endocrinology 132: 2009-2016. doi: 10.1210/endo.132.5.8477652. [View Article] [PubMed] [Google Scholar]
- Gillott DJ, Iles RK, Chard T (1996) The effects of beta-human chorionic gonadotrophin on the in vitro growth of bladder cancer cell lines. Br J Cancer 73: 323-326. [PubMed] [Google Scholar]
- Cole LA, Butler S (2011) Hyperglycosylated hCG, hCGβ and Hyperglycosylated hCGβ: interchangeable cancer promoters. Mol Cell Endocrinol 349: 232-238. doi: 10.1016/j.mce.2011.10.029. [View Article] [PubMed] [Google Scholar]
- Apaja PM, Tuusa JT, Pietilä EM, Rajaniemi HJ, Petäjä-Repo UE (2006) Luteinizing hormone receptor ectodomain splice variant misroutes the full-length receptor into a subcompartment of the endoplasmic reticulum. Mol Biol Cell 17: 2243-2255. doi: 10.1091/mbc.E05-09-0875. [View Article] [PubMed] [Google Scholar]
- Chambers AE, Stanley PF, Randeva H, Banerjee S (2011) Microvesicle-mediated release of soluble LH/hCG receptor (LHCGR) from transfected cells and placenta explants. Reprod Biol Endocrinol 9: 64. doi: 10.1186/1477-7827-9-64. [View Article] [PubMed] [Google Scholar]
- Johnson AM, O'Connell MJ, Messing EM, Reeder JE (2008) Decreased bladder cancer growth in parous mice. Urology 72: 470-473. doi: 10.1016/j.urology.2008.04.028. [View Article] [PubMed] [Google Scholar]
- Bertram JS, Craig AW (1972) Specific induction of bladder cancer in mice by butyl-(4-hydroxybutyl)-nitrosamine and the effects of hormonal modifications on the sex difference in response. Eur J Cancer 8: 587-594. [PubMed] [Google Scholar]
- Miyamoto H, Yang Z, Chen Y, Ishiguro H, Uemura H, et al. (2007) Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst 99: 558-568. doi: 10.1093/jnci/djk113. [View Article] [PubMed] [Google Scholar]
- Izumi K, Taguri M, Miyamoto H, Hara Y, Kishida T, et al. (2014) Androgen deprivation therapy prevents bladder cancer recurrence. Oncotarget 5: 12665-12674. doi: 10.18632/oncotarget.2851. [View Article] [PubMed] [Google Scholar]
- Dietrich K, Demidenko E, Schned A, Zens MS, Heaney J, et al. (2010) Parity, early menopause and the incidence of bladder cancer in women: a case-control study and meta-analysis. Eur J Cancer 47: 592-599. doi: 10.1016/j.ejca.2010.10.007. [View Article] [PubMed] [Google Scholar]
- Cantwell MM, Lacey Jr JV, Schairer C, Schatzkin A, Michaud DS (2006) Reproductive factors, exogenous hormone use and bladder cancer risk in a prospective study. Int J Cancer 119: 2398-2401. doi: 10.1002/ijc.22175. [View Article] [PubMed] [Google Scholar]
- Weibull CE, Eloranta S, Altman D, Johansson ALV, Lambe M (2013) Childbearing and the risk of bladder cancer: a nationwide population-based cohort study. Eur Urol 63: 733-738. doi: 10.1016/j.eururo.2013.01.005. [View Article] [PubMed] [Google Scholar]
- Davis-Dao CA, Henderson KD, Sullivan-Halley J (2011) Lower risk in parous women suggests that hormonal factors are important in bladder cancer etiology," Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the. American Society of Preventive Oncology 20: 1156-1170.[Google Scholar]
- Li D, Wen X, Ghali L, Al-Shalabi FM, Docherty SM, et al. (2008) hCG beta expression by cervical squamous carcinoma--in vivo histological association with tumour invasion and apoptosis. Histopathology 53: 147-155. doi: 10.1111/j.1365-2559.2008.03082.x. [View Article] [PubMed] [Google Scholar]
- Shah T, Srirajaskanthan R, Bhogal M, Toubanakis C, Meyer T, et al. (2008) Alpha-fetoprotein and human chorionic gonadotrophin-beta as prognostic markers in neuroendocrine tumour patients. Br J Cancer 99: 72-77. doi: 10.1038/sj.bjc.6604428. [View Article] [PubMed] [Google Scholar]
- Sheaff MT, Martin JE, Badenoch DF, Baithun SI (1996) beta hCG as a prognostic marker in adenocarcinoma of the prostate. J Clin Pathol 49: 329-332. [PubMed] [Google Scholar]
- Hotakainen K, Lintula S, Jarvinen R, Paju A, Stenman J, et al. (2006) Overexpression of human chorionic gonadotropin beta genes 3, 5 and 8 in tumor tissue and urinary cells of bladder cancer patients. Tumour Biol 28: 52-56. doi: 10.1159/000097703. [View Article] [PubMed] [Google Scholar]
- Gori S, Porrozzi S, Roila F, Gatta G, De Giorgi U, et al. (2005) Germ cell tumours of the testis. Crit Rev Oncol Hematol 53: 141-164. doi: 10.1016/j.critrevonc.2004.05.006. [View Article] [PubMed] [Google Scholar]
- Kuorelahti A, Rulli S, Huhtaniemi I, Poutanen M (2007) Human chorionic gonadotropin (hCG) up-regulates wnt5b and wnt7b in the mammary gland, and hCGbeta transgenic female mice present with mammary Gland tumors exhibiting characteristics of the Wnt/beta-catenin pathway activation. Endocrinology 148: 3694-3703. doi: 10.1210/en.2007-0249. [View Article] [PubMed] [Google Scholar]
- Zygmunt M, Herr F, Keller-Schoenwetter S, Kunzi-Rapp K, Münstedt K, et al. (2002) Characterization of human chorionic gonadotropin as a novel angiogenic factor. J Clin Endocrinol Metab 87: 5290-5296. doi: 10.1210/jc.2002-020642. [View Article] [PubMed] [Google Scholar]
- Michel RM, Aguilar JL, Arrieta O (2006) Human chorionic gonadotropin as an angiogenic factor in breast cancer during pregnancy. Med Hypotheses 68: 1035-1040. doi: 10.1016/j.mehy.2006.05.072. [View Article] [PubMed] [Google Scholar]

